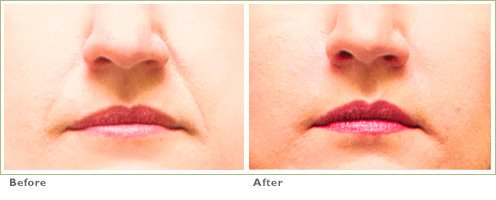
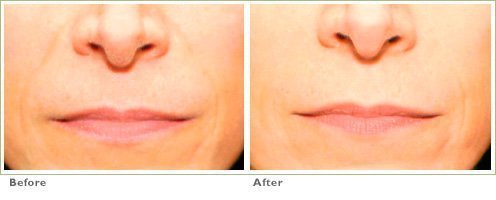
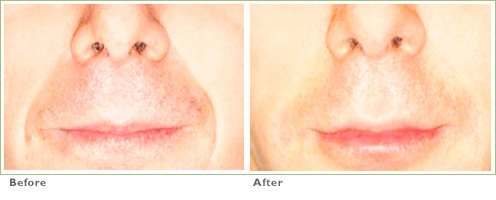
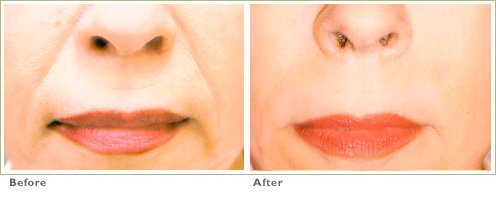
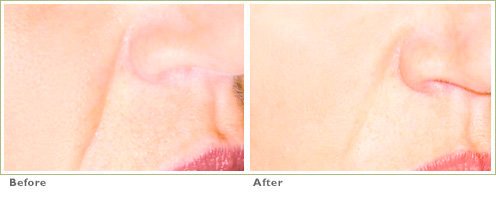
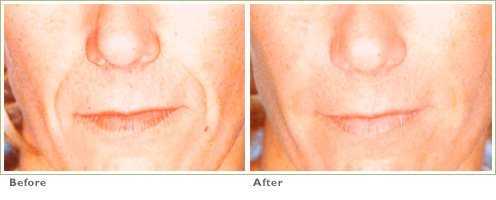
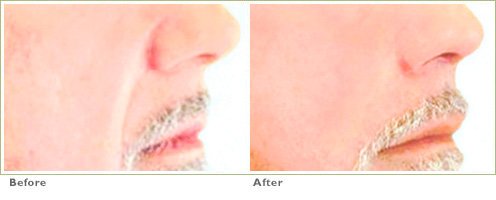
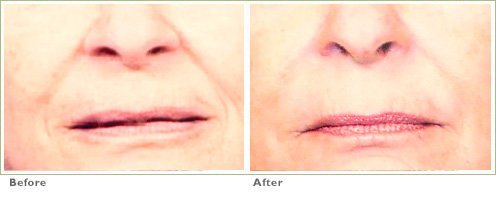
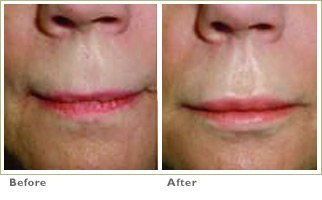
Frown Lines
About Radiesse
Why Choose RADIESSE?
We spend our entire lives defining who we are. From wardrobe to career choices, we decide how to make a name for ourselves. So, when the visible signs of aging take hold, threatening your very definition, it’s time to take back control and define yourself, your way.
RADIESSE is a viable option for those seeking help in smoothing their wrinkles. Long-lasting and highly satisfying to patients1,2, it is the first FDA-approved dermal filler for both face and hands.3,4
Collagen As Stimulated By RADIESSE
Collagen is the main structural protein in our skin–it’s what helps it feel and look tight, taught, and youthful. As we age, the production of collagen slows down, causing skin to sag.
RADIESSE is a collagen stimulator.5 It stimulates the body’s process of generating collagen and encourages growth, for a more refreshed appearance, so beauty can bloom.5,6

RADIESSE is comprised of calcium hydroxylapatite (CaHA) microspheres suspended in an aqueous gel carrier.5

Once injected, it provides immediate volume and correction and continues to work by stimulating the body to produce its own natural collagen for a long-lasting effect.5

Over time, the gel is absorbed and the body metabolizes the CaHA microspheres, leaving behind only your own natural collagen.5
What are RADIESSE® and RADIESSE® (+)?
RADIESSE® and RADIESSE® (+) are dermal fillers that are used for smoothing moderate to severe facial wrinkles and folds, such as nasolabial folds (the creases that extend from the corner of your nose to the corner of your mouth). RADIESSE® is also used for correcting volume loss in the back of the hands.
RADIESSE® and RADIESSE® (+) IMPORTANT SAFETY INFORMATION
Who should not use RADIESSE® or RADIESSE® (+)?
You should not use RADIESSE® or RADIESSE® (+) if you have an allergy to any component of the product, if you have a history of severe allergies, if you have a bleeding disorder, or if you are pregnant or breastfeeding. You should not use RADIESSE® (+) if you have an allergy to lidocaine or medicines like it.
What is the most important information I should know about RADIESSE® and RADIESSE® (+)?
One of the risks with using these products is unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious, and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. If you have changes in your vision, signs of a stroke (including sudden difficulty speaking, numbness or weakness in your face, arms, or legs, difficulty walking, face drooping, severe headache, dizziness, or confusion), white appearance of the skin, or unusual pain during or shortly after treatment, you should notify your health care practitioner immediately.
As with all procedures that involve an injection through the skin, there is a risk of infection.
Do not use RADIESSE® or RADIESSE® (+) if you have a skin infection until it has healed.
It is not known if RADIESSE® or RADIESSE® (+) is safe or effective in the lips, or in the area around the eyes.
The microspheres in RADIESSE® and RADIESSE® (+) can be seen in X-rays and CT Scans. It is very important that you tell your health care provider that you have had RADIESSE® or RADIESSE® (+) dermal filler.
If you have a history of herpes, you may experience a herpes breakout after receiving RADIESSE® or RADIESSE® (+).
Injection in the back of the hand may result in temporary difficulty performing activities. RADIESSE® may cause nodules, bumps or lumps in the back of the hand and can last up to 1 year.
You should minimize strenuous activity and avoid extensive sun or heat exposure for about 24 hours after treatment and until any swelling or redness has resolved.
What should I tell my doctor before using RADIESSE® or RADIESSE® (+)?
Tell your health care provider if you are taking blood thinners or medicines that can interfere with the clotting of blood, such as aspirin or warfarin. These medicines might make it more likely that you will experience bruising or bleeding at the injection site. Tell your health care provider if you have any diseases, injuries or disabilities of the hand, if you have a history forming large, raised scars or if you have had any other skin treatments such as skin peels.
What are the most common adverse events with RADIESSE® or RADIESSE® (+)?
The most common adverse events seen in clinical studies of RADIESSE® used in the hands include bruising, redness, swelling, pain, itching, nodules or bumps/lumps, difficulty performing activities, loss of sensation and other local side effects. The most common adverse events seen in clinical studies of RADIESSE® or RADIESSE® (+) used in the face include bruising, redness, swelling, pain, itching and other local side effects.
These are not all of the possible side effects with RADIESSE® or RADIESSE® (+). Merz collects information about adverse events seen with RADIESSE® and RADIESSE® (+) outside of clinical studies. These events are included in the RADIESSE® and RADIESSE® (+) Patient Information Guide based on an assessment of seriousness and potential causal relationship to RADIESSE® or RADIESSE® (+). Please see the Patient Information Guide available at www.radiesse.com for list of these events. Tell your health care provider about any side effects that bother you or do not go away.
Important: For full safety information, please visit www.Radiesse.com or call MyMerz Solutions at 1-844-469-6379
RADIESSE® and RADIESSE® (+) are available by prescription only.
References:
- Moers-Carpi M, Vogt S, Santos BM, Planas J, Vallve SR, Howell DJ. A multicenter, randomized trial comparing calcium hydroxylapatite to two hyaluronic acids for treatment of nasolabial folds. Dermatol Surg. 2007;33(suppl 2):S144-S151.
- Moers-Carpi MM, Tufet JO. Calcium hydroxylapatite versus nonanimal stabilized hyaluronic acid for the correction of nasolabial folds: a 12-month, multicenter, prospective, randomized, controlled, split-face trial. Dermatol Surg. 2008;34(2):210-215.
- RADIESSE® [Instructions for Use]. Franksville, WI: Merz North America, Inc.; 2016.
- RADIESSE [Instructions for Use for the dorsum of the hand]. Franksville, WI: Merz North America, Inc.; 2016.
- Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34(suppl 1):S64-S67.
- Yutskovskaya Y, Kogan E, Leshunov E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13(9):47-52.
Radiesse Gallery
Radiesse for Wrinkles & Aging Skin
Radiesse for Lips
*Results may vary.